Abstract
Background: The combination of a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and dexamethasone is the current standard induction therapy for myeloma. Daratumumab (Dara), a monoclonal antibody directed against CD38, is highly effective in treating myeloma and improves response rates and progression free survival (PFS) when added to a PI or IMiD. Recent studies have shown high efficacy rates with quadruplet regimens that contain these three drug classes, potentially allowing for limited duration therapy as well as reduction in the steroid doses. We designed this trial to examine the efficacy of the quadruplet regimen containing daratumumab, ixazomib (an oral proteasome inhibitor), lenalidomide and dexamethasone (Dara-IRd) given for fixed duration, and to evaluate impact of early discontinuation of steroids.
Patients and Methods: Patients with previously untreated MM who had measurable disease and adequate organ function were enrolled, irrespective of their autologous stem cell transplant eligibility (NCT03012880). The primary objective was to determine the rate of complete response to Dara-IRD combination. Treatment involved 28-day cycles consisting of ixazomib 4 mg days 1, 8, 15; lenalidomide 25 mg days 1-21, dexamethasone 40 mg, weekly and Dara 16 mg/kg, weekly for two cycles, every other week during cycles 3-6 and then every 4 weeks thereafter during induction (12-cycles) followed by Dara-ixazomib maintenance (maximum 24 cycles). Patients were enrolled into two sequential cohorts: Cohorts A (n=38) and B (N=40), with dexamethasone discontinued after two cycles in Cohort B. Transplant eligible patients were allowed to collect stem cells for future transplant anytime after 4 cycles. Serum immunofixation was assessed with mass spectrometry (MASS-FIX) and marrow MRD negativity by Euro flow (sensitivity of 1 in 105).
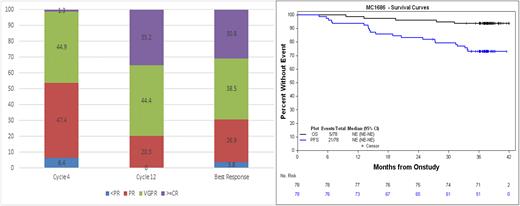
Results: Seventy-eight patients were enrolled; median age was 63.5 (range 33, 81), 55% were male. A third of the patients were high risk by mSMART criteria and ISS stage 1, 2, and 3 had 41%, 35%, and 24% of patients respectively. All patients have completed the treatment phase and those who have not gone off study continue on observation. Among the 40 patients who have gone off study, the reasons for discontinuation among patients in Cohorts A and B, respectively were: progression, 9 and 12; alternate therapy (most often transplant), 11 and 8. The best overall response on therapy was 96% including 31% CR, 39% VGPR. For the entire cohort, time to response was fast, median time to VGPR, CR or minimal residual disease negative (MRD) negativity was 4 cycles. Responses deepened over the duration of treatment as shown in the figure; 31% of patients achieved a marrow MRD negative status (28% CR and MRDneg). After a median follow up was 37.8 months, median PFS or OS has not been reached. Five patients enrolled on the study have died. A grade 3 or higher adverse event at least possibly attributed to the study drugs was seen in 56% of patients. The most common toxicities included fatigue, neutropenia, lymphopenia, peripheral neuropathy, diarrhea, and nausea. Stem cells were collected in 60 patients; median (range) of CD34 cells/kg 7.8 (2.8, 15.9) x 106, with plerixafor used in 88% of patients as per our standard of care "on-demand” approach based on blood CD34 count.
Conclusion: Dara-IRd is an active regimen in newly diagnosed MM, with high overall rates of response and deep and durable responses with nearly a third of the patients being MRDneg (Mass Spec based blood testing and flow based MRD), that improved over time with therapy. The toxicities were manageable, with dose modifications. All patients were able to collect stem cells when required, though the majority needed plerixafor. This study demonstrates excellent outcomes with limited duration quadruplet regimen without planned upfront autologous stem cell transplant and no maintenance till progression.
Disclosures
Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Muchtar:Protego: Consultancy; Janssen: Honoraria. Dingli:Alexion Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Sanofi S.A.: Consultancy. Kourelis:Novartis: Research Funding. Lacy:Celgene: Research Funding. Dispenzieri:Oncopeptides, and Sorrento: Other: Data monitoring safety committee; Janssen: Membership on an entity's Board of Directors or advisory committees; Alynlam, Pfizer, Takeda, and BMS: Research Funding. Leung:Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Kapoor:Sanofi: Honoraria, Research Funding; X4 Pharmaceuticals: Honoraria; Regeneron: Research Funding; Amgen: Research Funding; Ichnos: Research Funding; Loxo: Research Funding; Karyopharma: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Research Funding; Casma: Honoraria; Pharmacyclics: Honoraria; Imedex: Honoraria; GSK: Honoraria; Cellectar: Honoraria; Oncopeptides: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal